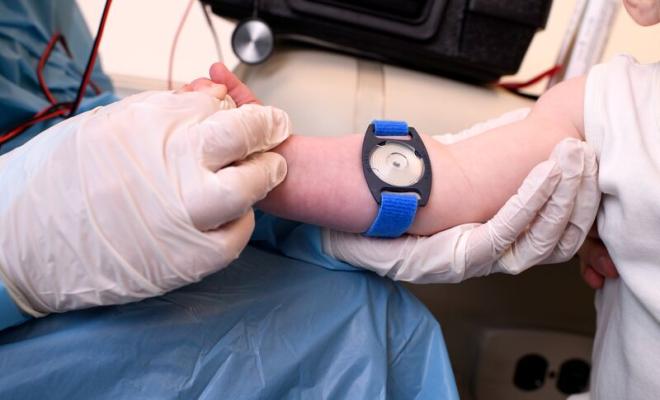
Sweat Test Clinical Care Guidelines: Executive Summary
LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ, Cystic Fibrosis Foundation. Diagnostic Sweat Testing: The Cystic Fibrosis Foundation Guidelines. J Pediatr. 2007 Jul;151(1):85-9.PMID: 17586196
Measurement of sweat chloride by quantitative pilocarpine iontophoresis remains the standard test for diagnosing cystic fibrosis (Farrell 2017). The procedures for sweat collection and analysis must be rigorously controlled to ensure correct interpretation of the test. The widespread implementation of newborn screening (NBS) programs has resulted in more testing of asymptomatic infants at an early age.
Obtaining a sufficient volume of sweat on newborns can be challenging, and requires careful adherence to laboratory procedures to achieve a quantity not sufficient (QNS) rate of 10 percent or less for infants 3 months and younger (LeGrys 2010). In addition to diagnosis, sweat chloride concentrations are sometimes used as a biomarker of cystic fibrosis transmembrane conductance regulator (CFTR) activity for evaluating CFTR-modulating drugs.
Since the publication of the 2007 guidelines, the Clinical & Laboratory Standards Institute (CLSI) document for sweat testing has been updated to include information about method validation and obtaining a sufficient sample volume (CLSI 2009). The new diagnosis guidelines state that a sweat chloride concentration of 60 millimoles per liter (mmol/L) or more indicates a diagnosis of CF, and a concentration of less than 30 mmol/L indicates that CF is unlikely regardless of age (Farrell 2017).
Methodology
The recommendations were developed by consensus based on expert opinion and a review of the literature.
Recommendations
Collection |
|
| Recommendations | Evaluation of the Evidence |
| 1. Patients must be more than 48 hours old because sweat electrolytes can be transiently elevated in the first 24 hours of life. | Expert Committee |
| 2. Iontophoresis equipment must be battery powered and regularly inspected to ensure safety. | Expert Committee |
| 3. Sweat must be collected from arms or legs only. This prevents the iontophoresis current from stimulating the heart. | Expert Committee |
| 4. Duplicate collection and analysis is recommended due to underlying variability in the test and the potential for an insufficient sample. | Expert Committee |
|
5. Sweat must be collected on gauze, filter paper, or in a Macroduct® coil, using techniques to minimize evaporation and contamination. The sample must be appropriately labeled for patient identification throughout collection and analysis.
|
Expert Committee |
Analysis |
|
| Recommendations | Evaluation of the Evidence |
| 6. Sweat must be quantitatively analyzed for chloride using an approved method to include at least two levels of controls. | Expert Committee |
| 7. Unacceptable methods for diagnosis include measurement of sweat conductivity, osmolality, sodium, or potassium; use of point of care skin precipitation patches; and direct application of the chloride electrode to skin. | Expert Committee |
| 8. Duplicate samples must be analyzed separately and not pooled. | Expert Committee |
| 9. Insufficient samples should not be analyzed. | Expert Committee |
| 10. The lower limit of detection must be ≤10 mmol/L. The upper end of reportable results should be no more than 160 mmol/L, as this is beyond the physiologic range. | Expert Committee |
Interpretation |
|
| Recommendations | Evaluation of the Evidence |
| 11. Sweat chloride ≥60 mmol/L is consistent with a diagnosis of cystic fibrosis, although some other conditions can cause elevated sweat chloride. | Expert Committee |
| 12. All positive tests must be confirmed on a separate date or with an independent diagnostic method (e.g., genetic testing). | Expert Committee |
Quality Assurance and Testing Availability |
|
| Recommendations | Evaluation of the Evidence |
| 13. Centers must maintain <5 percent incidence of QNS tests for patients older than 3 months. | Expert Committee |
| 14. Sweat testing must be performed on a sufficient number of patients by well-trained personnel. | Expert Committee |
| 15. Laboratories must maintain successful performance on College of American Pathology (CAP) proficiency testing. | Expert Committee |
| 16. New personnel must demonstrate competency every six months for the first year and annually thereafter. | Expert Committee |
| 17. Sweat testing must be available two days per week. Wait times for sweat tests should be less than two weeks. | Expert Committee |
| 18. All tests should be evaluated by the center director and lab director and become part of the laboratory's ongoing quality improvement plan (CLSI 2009). | Expert Committee |
New Issues
- Normal sweat chloride concentration is 29 mmol/L or less (Farrell 2017).
- Intermediate sweat chloride concentration is 30-59 mmol/L (Farrell 2017). Intermediate sweat chloride values have been documented in genetically proven cystic fibrosis.
- Newborns with a positive CF newborn screen should have the test performed bilaterally when the infant weighs more than 2 kilograms (kg), and is at least 36 weeks of corrected gestational age. These measures increase the likelihood of collecting an adequate sweat specimen (Farrell 2017).
- Newborns greater than 36 weeks' gestation and more than 2 kg body weight who have a positive CF newborn screen or a positive prenatal genetic test should have sweat chloride testing performed as soon as possible after 10 days old, ideally by the end of the neonatal period (Farrell 2017).
- For infants with presumptive CF identified through NBS, CF treatment should not be delayed while efforts to establish a diagnosis of CF are initiated (Farrell 2017).
- Sweat chloride analysis should be performed within a few hours of sweat collection, and the results and interpretations should be reported to clinicians and parents or patients as soon as possible and certainly on the same day (Farrell 2017).
- Patients who have intermediate sweat chloride concentrations should have repeat tests within 1-2 months. Genetic testing should be offered. Ancillary tests, such as stool elastase, should be considered (Ren 2017).
Unanswered Questions
- How can the QNS rate for infants be reduced?
- What is a reasonable QNS rate for infants less than 1 month old?
- How can the higher rates of QNS be decreased in newborns and premature infants?
- When is the optimal time to sweat test an infant with a positive newborn screening test?
- Can collection devices and techniques be further optimized for testing infants?
- When should symptomatic children with negative newborn screening results be sweat tested?
Further Reading
Relevant manuscripts published after the original guidelines are listed below. These manuscripts have not been reviewed or endorsed by the guidelines committee.
- Farrell PM, White TB, Ren CL, Hempstead SE, Accurso F, Derichs N, Howenstine M, McColley SA, Rock M, Rosenfeld M, Sermet-Gaudelus I, Southern KW, Marshall BC, Sosnay PR. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr. 2017 Feb;181S:S4-S15.e1.PMID: 28129811
- LeGrys VA, McColley SA, Li Z, Farrell PM. The need for quality improvement in sweat testing infants after newborn screening for cystic fibrosis. J Pediatr. 2010 Dec;157(6):1035-7. Epub 2010 Sep 16.PMID: 20843526
- CLSI. Sweat Testing: Sample Collection and Quantitative Chloride Analysis: Approved Guideline-Third Edition. CLSI document C34-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2009.
- Ren CL, Borowitz DS, Gonska T, Howenstine MS, Levy H, Massie J, Milla C, Munck A, Southern KW. Cystic Fibrosis Transmembrane Conductance Regulator-Related Metabolic Syndrome and Cystic Fibrosis Screen Positive, Inconclusive Diagnosis. J Pediatr. 2017 Feb;181S:S45-S51.e1.PMID: 28129812
Use of These Guidelines
The CF Foundation intends for this executive summary of its guideline to summarize the published guideline. The published guideline summarizes evidence, and provides reasonable clinical recommendations based on that evidence, to clinicians, patients, and other stakeholders. Care decisions regarding individual patients should be made using a combination of these recommendations, the associated benefit-risk assessment of treatment options from the clinical team, the patient's individual and unique circumstances, as well as the goals and preferences of the patients and families that the team serves, as a part of shared decision-making between the patient and clinician.
This executive summary was prepared by:
Anthony J. Fischer, MD, PhD, (University of Iowa Children's Hospital) and Vicky A. LeGrys, DA, (University of North Carolina at Chapel Hill)
The guidelines were published in July 2007, they were reviewed in July 2021 and it was determined that no update is needed at this time.