What Is a Study Protocol?
A protocol is a plan that explains how the trial will work, what will be done during the trial, and why. The trial sponsor, often the pharmaceutical company that develops the therapy or medication, designs the protocol for the clinical trial. While designing a protocol, sponsors often work with the Foundation to get feedback from research experts and people with CF.
Key information in a protocol includes:
- How many people will participate
- Who is eligible to participate
- What tests participants will get and how often
- What type of data will be collected
- How long the study will last
- What medication and dosages participants will receive, if appropriate
The protocol must go through many layers of review before a study can begin. Once a protocol is approved, the sponsor chooses principal investigators to run the trial. Each investigator follows the same protocol to ensure that the study is conducted in the same way at each participating center.
Ensuring Trials are Unbiased
Bias refers to human choices or other factors (unrelated to the protocol) that might affect a trial's results. For example, if doctors could choose which people to assign to comparison groups in a study, some might assign sicker people to the treatment group and healthier people to the placebo group. The doctors might not even realize they are doing this, and it could affect trial results.
Researchers can avoid bias by designing a study in a certain way:
- Randomization helps ensure that researchers don't introduce bias into the trial. In many clinical trials that test the effectiveness of a medication, half of the participants receive the medication in question. The other half receive a placebo, which contains no medication. Randomization involves assigning participants to these comparison groups by chance, rather than choice.
- Blinding can also help avoid bias. In a blinded trial, researchers will not know which participants are receiving treatment and which ones are receiving a placebo.
Who Can Participate?
All clinical trials have guidelines about who can join, known as inclusion and exclusion criteria. These criteria exist to ensure that the trial results are accurate and useful, and also to protect participant's safety. Criteria are based on factors such as:
- Age
- CFTR mutation
- Current state of health
- Previous treatment history
- Other medical conditions
The criteria depend on the type of trial. For example, if researchers are testing how well a particular antibiotic works in fighting Pseudomonas aeruginosa, then the trial would have inclusion criteria specifying that only people with CF who are infected with that bacterium can join the trial. Those who do not have that bacterial infection would be excluded from the trial.
Another example is the age requirement for a trial. Drugs work differently in kids than they do in adults. To reduce the risk for younger kids with CF who participate in a trial, drugs must first be shown to be safe and effective in adults with CF. Clinical trials for a new drug will usually start in adults 18 years and older before moving down to younger age groups.
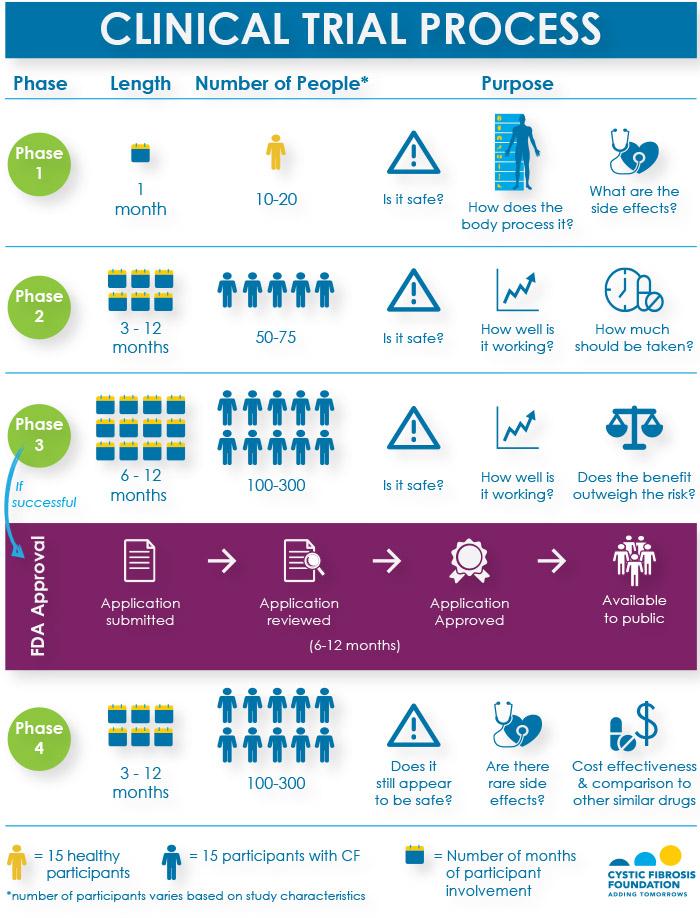
The Four Phases of Clinical Research
For any new drug to receive approval by the U.S. Food and Drug Administration (FDA) and become available to the public, it must pass through three phases of interventional clinical trials to show that it is safe and effective in treating the disease. If the FDA approves the drug, researchers will continue to monitor for safety and effectiveness in what is known as a Phase 4 study. The infographic below shows a breakdown of the questions that researchers try to answer, the number of participants needed, and the length of participation for each phase of research.
Please note that the length of time refers to the time it takes to participate in a trial, not the entire length of the phase. It takes additional time to enroll participants and to process and analyze the results of each phase. All told, it typically takes 10 to 14 years from the time a drug is discovered in a laboratory to its possible approval by the FDA for people with CF.
About Study Sponsors
Clinical research can be sponsored (i.e., paid for) in part or entirely by any number of organizations or individuals. For example, medical institutions, universities, foundations, voluntary groups, drug companies, and federal agencies, such as the National Institutes of Health (NIH), all sponsor research.
The CF Foundation is the primary supporter of CF research in the United States. Nearly all approved CF therapies available today were made possible because of research funded by the Foundation.
We facilitate and financially support clinical trials through our Therapeutics Development Network (TDN), which is made up of CF care centers with an expertise in clinical research.