For the last seven years, I've been an active participant in clinical research for cystic fibrosis. It is something I'm incredibly passionate about. Ongoing research aims to cure CF, while also improving current methods of treatment and enhancing the quality of life for those of us living with this disease. Having benefited from therapies that were developed and tested by those before me, participating in clinical research feels like a small way I can pay it forward.
The benefits of participating in research are numerous. I get to see certain members of my CF care team more frequently. Having these additional visits has helped forge deeper connections and trust between my care team and me. Study visits also make it possible to keep a vigilant eye on my health. I am always compensated for my time and effort. Also, I genuinely love science and research, so being able to take part in the advancement of those things makes me giddy.
When this pandemic began and all my health care appointments switched to a virtual platform, I immediately wondered what this would mean for research.
I wasn't enrolled in a clinical trial, but I knew that it would make future enrollments tricky. When, months later, my study coordinator reached out with an offer to begin a new trial, I was admittedly a little nervous.
The proposed study was a Phase 1 trial. In my experience, Phase 1 trials are generally short-term, but more complex and demanding than later phases. For this study, I'd need to be seen at my hospital a total of six times. I hadn't stepped foot into a hospital for the better part of a year, so the idea was more than a little nerve-wracking.
Despite my initial uncertainty, I agreed to be seen for a screening visit. First, I needed to be tested for COVID-19. I did this at a local drive-through testing site. Once I arrived at the hospital, I immediately noticed some differences: There were spaces marked on the ground encouraging people to keep their physical distance, hand-sanitizing stations set up throughout the entrance and lobby, and masked attendants at the doors inquiring about symptoms and giving instructions to each person who entered.
I'd never seen the hospital so empty. Many chairs in the lobby had been removed, leaving considerable space between them. Everywhere I looked -- on the walls, in the elevator, and even in the bathroom stall -- there were signs reminding people to stay masked and stand 6 feet apart.
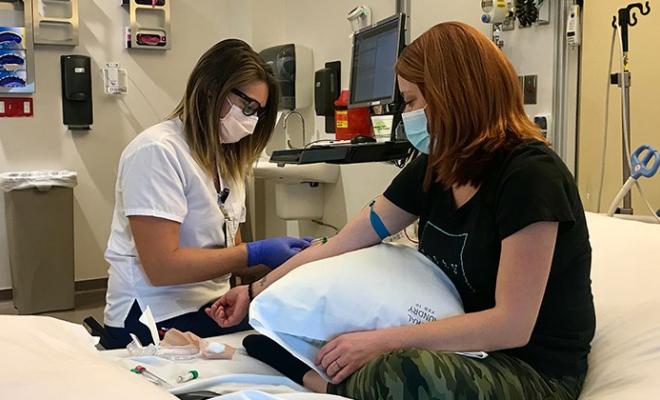
My study coordinator met me in the lobby, and we walked directly to a room. There was no check-in process or time spent in waiting areas. During my appointment, I came into contact only with the necessary people: my study coordinator, a nurse, and my doctor. Everyone was masked the entire time, and all three of them also wore eye protection.
I knew that I'd be doing pulmonary function tests (PFTs), but wasn't sure how that would work, because it is an aerosol-generating procedure that is considered higher risk for transmission of COVID. My study coordinator was the only other person in the room at the time of testing. During the procedure, she wore a fitted N-95 mask, gown, and goggles. A giant, portable HEPA filter was brought into the room and was running both during PFTs and for a significant amount of time afterward.
By the end of the appointment, I breathed a sigh of relief. After seeing all that was being done to keep both patients and staff safe, I no longer had any hesitations or concerns about how the rest of the study would go.
Before each appointment, I easily got tested for COVID and the same safety protocol was followed each time I was seen at the hospital.
Before my last scheduled visit, I developed a sinus infection. Since I was getting tested for COVID weekly, we had no reason to believe my symptoms were something more concerning. Even still, we canceled the appointment just to be safe. I was able to drop a sputum sample off at a local lab (utilizing local labs has been unbelievably helpful during this pandemic), start treatment at home, and reschedule the appointment for a time when I was feeling better. My final appointment fell outside the scheduled window, but it seems study sponsors are well aware of the fact that this pandemic has added a new layer of unpredictability to CF research.
My experience with research in the time of COVID has been a bit different, but it's still been great. I've come away with an even better appreciation for the tireless work of the medical and scientific communities. Even a global pandemic can't stop them! Research is no less important now than it was before our world was turned upside down by a virus. It's comforting to know that so much is being done to protect everyone involved as we continue doing this important work together.
Interested in sharing your story? The CF Community Blog wants to hear from you.