We continue to make tremendous progress towards these goals. Last year the U.S. Food and Drug Administration (FDA) expanded the approval of three CFTR modulators, including Trikafta®, using in vitro data to people with certain mutations. It also expanded Kalydeco® to infants as young as four months. However, we will not rest until everyone has a therapy that addresses the underlying cause of their disease.
With this commitment in mind, the Foundation launched our Path to a Cure, an ambitious research initiative to accelerate treatments for everyone with CF and ultimately deliver a cure. We intend to allocate a half billion dollars to the effort through 2025 and have already seen an 180% increase in funding for this area of research from 2018.
It is also critical to continue to develop new and improved treatments for complications from the disease. We are building on the current momentum, funding an innovative research portfolio and collaborating with top scientists from around the world to deliver the next generation of transformative breakthroughs in CF. To ensure the advancement of potential therapies, we provide research funding and expertise to draw the best scientific minds and technologies into CF. Last year we held more than 230 meetings with over 120 companies — 61 of which were new to CF — and are currently funding nearly 50 different industry programs.
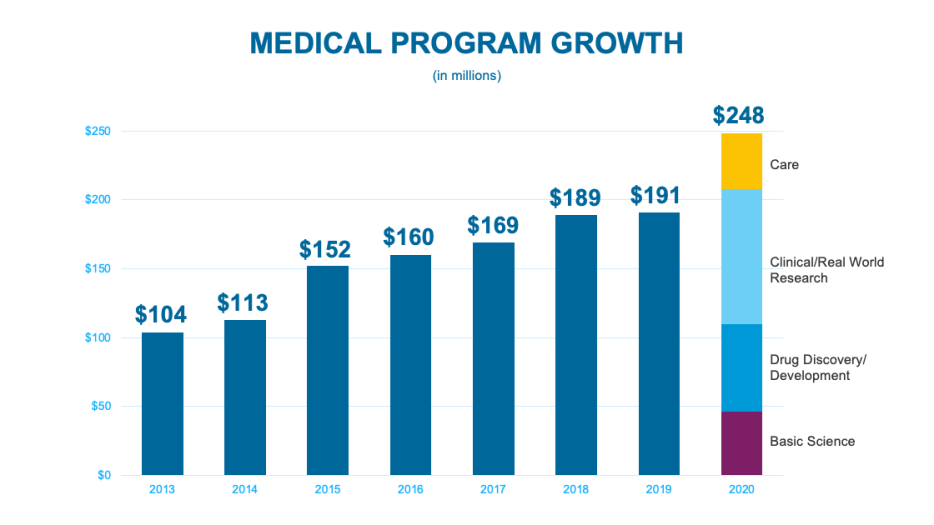
Despite the many challenges posed by the pandemic, in 2020 the CF Foundation funded approximately $248 million in laboratory research, preclinical drug development, clinical and real-world research, and high-quality, specialized care and training — more than at any other time in the history of the Foundation and nearly a third more than just the previous year.
Our Investment in Research
The CF Foundation spent a total of $218.1 million on research and development as well as the CF Foundation Therapeutics Lab in 2020.
***
Reference to any specific product, process, or service does not necessarily constitute or imply its endorsement, recommendation, or favoring by the Cystic Fibrosis Foundation. The appearance of external hyperlinks does not constitute endorsement by the Cystic Fibrosis Foundation of the linked websites, or information, products, or services contained therein.
Information contained on this site does not cover all possible uses, actions, precautions, side effects, or interactions. This site is not intended as a substitute for treatment advice from a medical professional. Consult your doctor before making any changes to your treatment.
FDA-approved drug information is available at dailymed.nlm.nih.gov/dailymed.