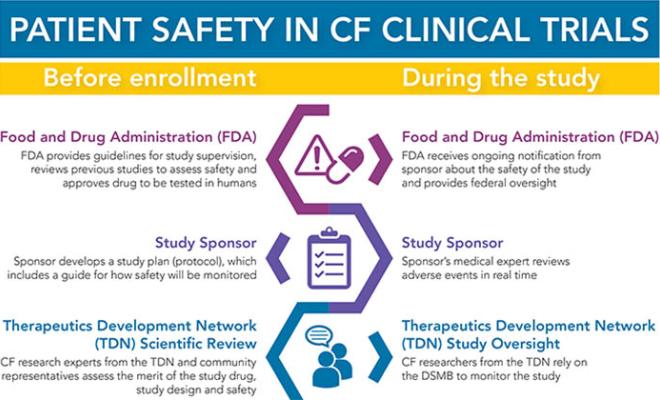
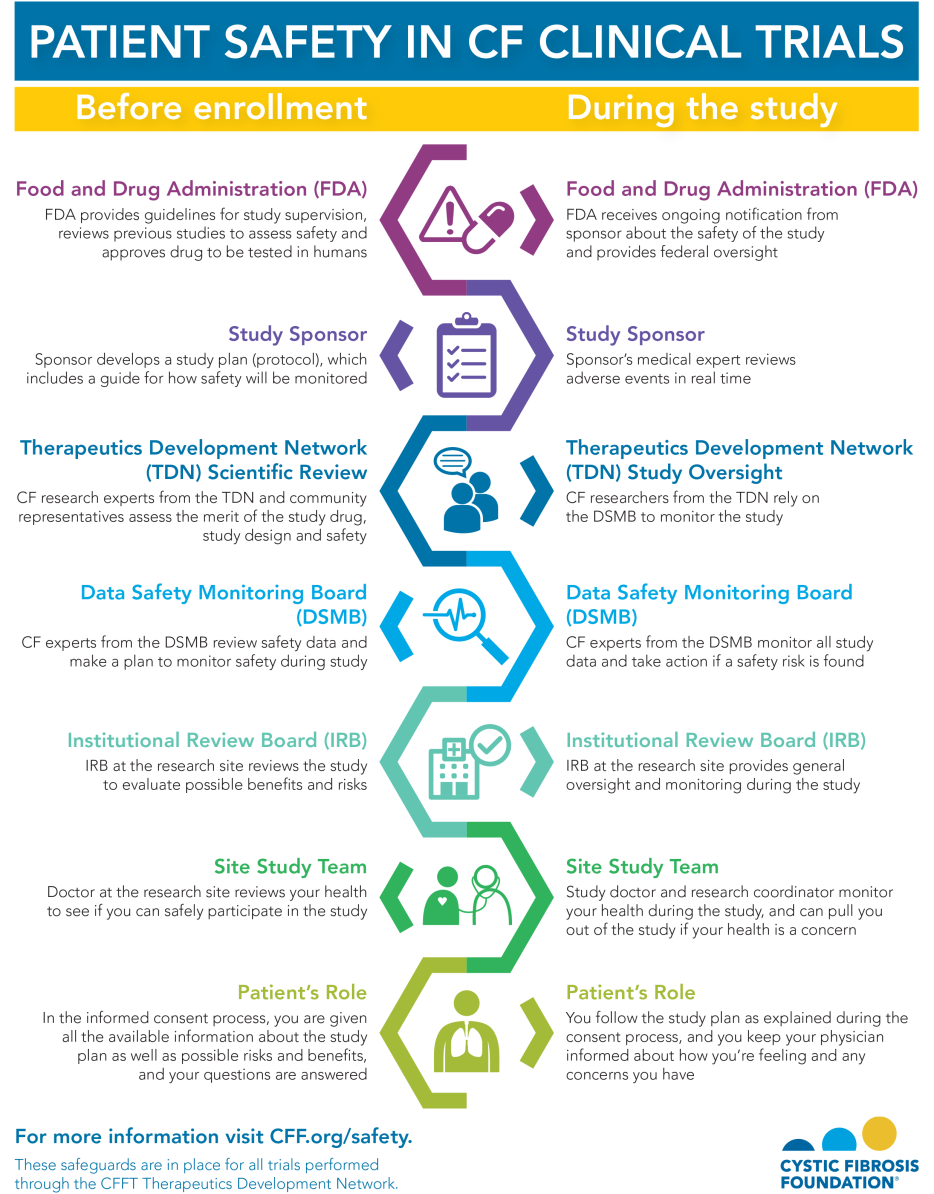
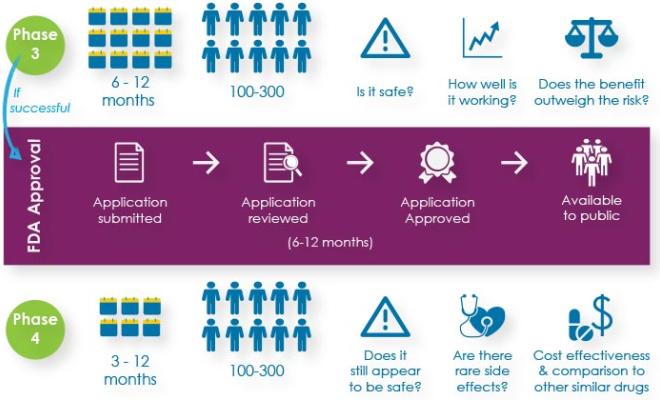
The participation of people with cystic fibrosis in research is crucial to the development of new therapies and in better understanding how to treat the disease. And nothing is more important than protecting their safety. That is why the Cystic Fibrosis Foundation decided early on to create its own specialized review committees and independent Data Safety Monitoring Board with CF experts to review the safety of clinical trials.
Every trial done through the Cystic Fibrosis Foundation Therapeutics Development Network must go through numerous layers of review to protect the safety of study participants. Check out the graphic below for a breakdown of what happens before a study begins and how safety is monitored throughout the study.
Learn more about how patient safety is prioritized in CF studies.
Interested in participating in a research study? Check out our Clinical Trial Finder.