This is an exciting time in cystic fibrosis clinical research. Not only are there several clinical trials of therapies to treat the underlying cause of CF, but there are also many trials to treat key symptoms of CF and improve quality of life. These include studies of antibiotics and anti-inflammatories to treat lung infections and inflammation, as well as treatments to improve nutrition and digestion.

There have never been more opportunities to help develop new drugs for cystic fibrosis than there are today. When you are deciding whether to participate in a clinical trial, it's important to know the potential risks and benefits.

Clinical trials that test potential drugs and therapies in people with cystic fibrosis are a major part of CF research. They take place at Cystic Fibrosis Foundation-accredited care centers all over the United States and enroll people with CF of all ages.

Once your clinical trial is over, you can follow its progress online or follow up with your care team to find out about results. Learn how to use the Clinical Trial Finder and Drug Development Pipeline to track your trial and stay informed about current research.

The Drug Development Pipeline features an extensive list of drugs that are in development or approved for treating cystic fibrosis.

Have questions about clinical trials? The Clinical Trial Navigator is a person who can help you get the answers you need.
Stay up-to-date on the latest clinical studies as they open for enrollment and publish results. Sign up to receive updates on all studies as they become available, or customize your alerts based on the studies that are important to you.
People with cystic fibrosis and their family members know CF better than anyone, and Community Voice provides opportunities for you to actively shape research and programs for the CF community.
BLOG
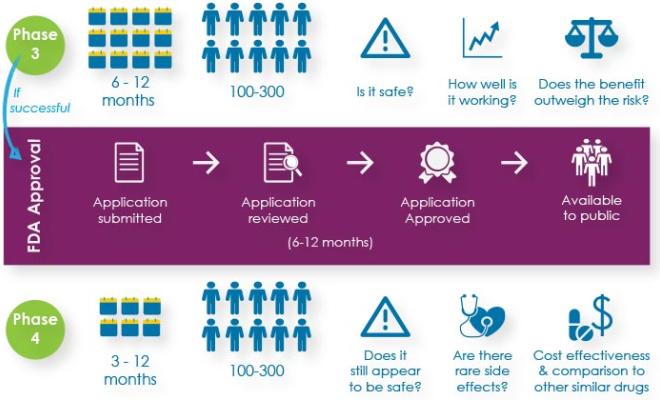
Ever wondered what it takes for a potential cystic fibrosis drug to become approved by the U.S. Food and Drug Administration? Here's an explanation of the four phases of clinical research.

BLOG
Sr. Vice President for Policy and Community Affairs Mary Dwight reflects on the passage of the Ensuring Access to Clinical Trials Act (EACT) and what it means for the community.

BLOG
Participating in clinical trials can be scary, but the sense of empowerment you get from knowing that you are contributing to a cure outweighs any second thoughts.