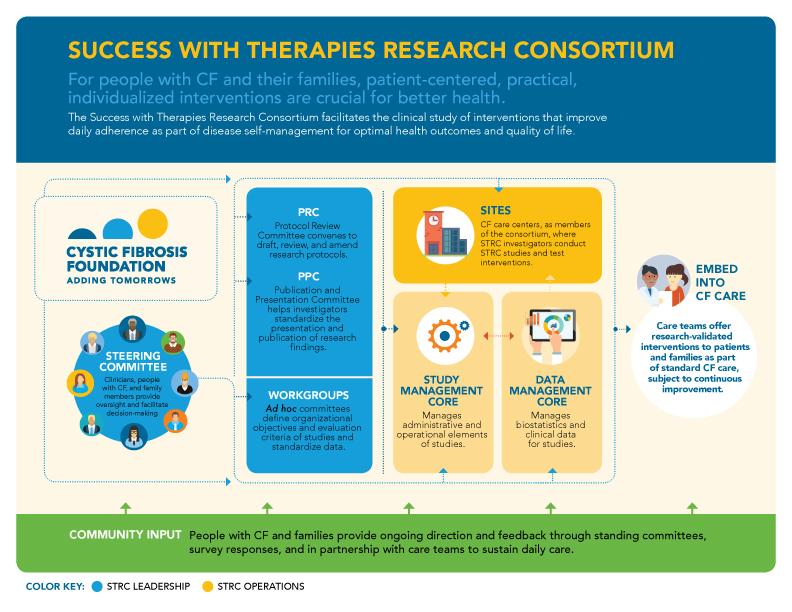
The Success With Therapies Research Consortium
The Success with Therapies Research Consortium facilitates the clinical study of interventions to improve day-to-day adherence and cystic fibrosis disease self-management to optimize health outcomes and quality of life.
Taking care of CF in everyday life is not always easy. Everyone has times when it is harder to complete treatments. Yet, recent research has shown that consistently managing a daily treatment plan is crucial for better health; it is associated with better lung function, fewer pulmonary flare-ups, and even lower health care costs.
Recognizing how hard CF daily care can be and the importance of keeping up with therapies, the Cystic Fibrosis Foundation created Partnerships for Sustaining Daily Care (PSDC). PSDC is a strategic initiative to support CF care by encouraging discussion between care teams and people with CF and their families, to identify what gets in the way of daily care and find solutions together.
The Success with Therapies Research Consortium (STRC) is a part of this initiative and aims to test interventions that are effective, practical, measurable, scalable, and sustainable. Formed in 2014, it is co-chaired by Kristin Riekert, PhD, from Johns Hopkins University, and Gregory Sawicki, MD, MPH, from Boston Children's Hospital. Drs. Riekert and Sawicki lead a group of investigators to identify innovative ways to support people with CF in managing their disease. STRC investigators have diverse backgrounds in medicine, psychology, clinical research, and pharmacy, and they represent adult and pediatric CF programs throughout the United States.
CF Community Input
People with CF and their families are involved at all levels of the STRC, including:
- Consulting on research questions, study designs, and protocols.
- Consulting on survey development.
- Co-chairing working groups.
- Serving as co-investigators on studies.
- Co-authoring manuscripts.
- Presenting at STRC and NACFC meetings.
The CF community makes its biggest impact through the Steering Committee. The Steering Committee oversees and governs STRC operations and studies. Four community members serve on the Steering Committee, two adults with CF and two parents of children with CF — serve on the Steering Committee. During their 3-year terms, they provide real-world insight into STRC functions.
Learn how people with cystic fibrosis and families are partnering with researchers to develop ways to make sustaining daily care easier.
The STRC investigators are supported by a team made up of three organizations:
The Study Management Core at Boston Children's Hospital manages key administrative and operational elements of STRC studies
- The Data Management Core at the Johns Hopkins Adherence Research Center is responsible for biostatistics and clinical data management for STRC studies
- The Operations Core at the Cystic Fibrosis Foundation is responsible for central operations and communications for the STRC
STRC Research
By developing and validating interventions and tools that work in the real world, these solutions are more likely to become a part of a care team's regular clinical practice and integrated into the daily care routines of people with CF and their families without adding to their treatment burden. As part of its process, the STRC is developing a standard set of measures, which will enable investigators to evaluate a single study, as well as reliably compare between studies. These measures address barriers to daily CF care, intricacies of prescribed treatment regimens, perceived adherence, and knowledge of prescribed treatments. For more information on these measures, please contact STRC@cff.org.
The current STRC studies are:
- Qualitative Understanding of Experience with the SIMPLIFY Trial (QUEST): This study seeks to understand the experiences of people with CF participating in the SIMPLIFY study. Through interviews with SIMPLIFY participants, we will examine opinions on treatment withdrawal research, perceived treatment burden, and how behaviors toward treatments may change after participating in a treatment withdrawal study.
- Daily Care Check-In (DCC): Based on feedback from a multidisciplinary group of clinicians and CF community members, the Barriers Screening Tool was reframed as the DCC and serves as a guide for ongoing conversations about daily CF care between clinicians, people with CF, and their families. Several care teams will be participating in a two-phase challenge during which they will focus on how best to implement the DCC in clinic and the quality of conversations generated by the responses to the DCC.
- Video-Based Tele-Coaching: This study seeks to use patient, parent, and care team feedback to design a novel, patient-centered, and practical tele-coaching intervention to enhance adherence in adolescents and young adults with CF.
- Medication Planning Mobile App: This study will evaluate the effectiveness of MedActionPlan® (MAP). MAP is a personalized, web-based mobile application that encourages self-management by providing adherence reminders, reinforcing education about therapies, and allowing people with CF to self-monitor their goals in lung function and weight tracking.
STRC is in the early phases of planning for a study on nutrition in adults with advanced CF lung disease and a study on mobile health (mHealth), which was identified as a key area of interest by the CF community.
The completed STRC studies are:
- Barriers Screening Tools: This study sought to identify barriers to daily CF care at the individual and system levels, and then to create standardized tools to help people with CF and clinicians identify these barriers to then offer interventions that are tailored to the person’s unique strengths and challenges.
- Web-Based Nutrition Tool: This was the first of a two-phased study. Feedback from care teams and parents of children with CF informed adaptations to an existing, in-person behavioral and educational intervention. The result was a web-based tool that was then integrated into clinical care so that care teams and parents could work together to manage nutrition for children with CF. The second phase was a randomized study in which use of the web-based tool was compared to the current standard of care.
Currently, requests for proposals are open only to STRC investigators. If you are interested in learning more, please contact STRC@cff.org.
***
Reference to any specific product, process, or service does not necessarily constitute or imply its endorsement, recommendation, or favoring by the Cystic Fibrosis Foundation. The appearance of external hyperlinks does not constitute endorsement by the Cystic Fibrosis Foundation of the linked websites, or the information, products, or services contained therein.
Information contained on this site does not cover all possible uses, actions, precautions, side effects, or interactions. This site is not intended as a substitute for treatment advice from a medical professional. Consult your doctor before making any changes to your treatment.
FDA-approved drug information is available at www.dailymed.nlm.nih.gov/dailymed.