Looking back on it, 2017 has been an amazing year in science for cystic fibrosis not only because of the approved expansion of ivacaftor (Kalydeco®) for 28 more mutations, but also because of the advancements made in new therapies for those who don't have a treatment for the underlying defect in CF.
“We will look back at 2017 as the time when highly effective next-generation CFTR modulator therapy went from something we hoped for to something we fully expect to occur,” said Michael P. Boyle, M.D., senior vice president of therapeutics development at the Cystic Fibrosis Foundation.
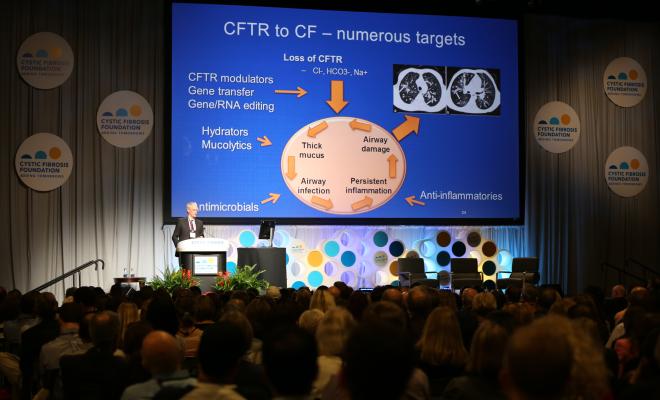
Dr. Boyle covered these recent drug developments and gave us insight into the potential impact of a new technique called theratyping, the process of matching CFTR modulators to individual mutations, during the first plenary, Matching Medicines With Mutations, at the 31st annual North American Cystic Fibrosis Conference in Indianapolis. The second speaker of the plenary, Philip Thomas, Ph.D., who holds the Ruth S. Harrell Professorship in Medical Research at The University of Texas Southwestern Medical Center in Dallas, explained how theratyping will enable the lab to play a critical role in the expanded use of therapies. (Watch a recording of the full session here.)
Dr. Boyle ticked off the list of this year's achievements:
- In March, two late-stage clinical trials of tezacaftor/ivacaftor showed positive results for those with two copies of the F508del mutation. The FDA will decide in February whether to approve the drug.
- In July, promising preliminary results came in from next-generation trials, which lay the groundwork for triple combination therapies that can help those with a single copy of the F508del mutation. Next-generation Phase 3 clinical trials should begin in mid-2018. Other companies also are planning Phase 1 trials for additional modulators next year.
- The U.S. Food and Drug Administration (FDA) approved the use of ivacaftor for 23 residual function mutations in May and five splice mutations in August.
What was remarkable about the latest accomplishment is that for the 23 residual function mutations, the FDA relied partially on lab data -- essentially how cells affected by these mutations responded to ivacaftor in lab experiments -- in conjunction with data from clinical trials, Dr. Boyle said. Normally, the FDA approves drugs primarily based on clinical trials data.
The FDA decision to use theratyping data advances the era of personalized medicine, defining a pathway to bring existing medications to people who have mutations so rare that it is not possible to conduct a clinical trial. This is significant because more than 1,000 CF mutations occur in five people or less.
Dr. Thomas, who was awarded the 2017 Paul di Sant'Agnese Distinguished Scientific Achievement Award at this year's NACFC, has dedicated his decades-long career to the science behind theratyping. He said he used to feel a little embarrassed when people asked him what he was working on, and he had to tell them he was still working on CF research. He thought he should have made much more progress.
However, today, speaking to the tremendous progress, Thomas said, “In the last three years, I have to tell you that I am proud I am working on CF."
He explained how the theratyping process is now coming to fruition: Researchers are testing approved modulators, which have been deemed safe in people with CF, on cells in the lab. They look for certain biological changes (called biomarkers) in the lab that signal that a drug is working and try to tie those results to improvements in the health of patients taking the drug.
Then, researchers use these same biomarkers to evaluate cells with very rare mutations, and try to predict whether the drug will work in people who have those mutations.
After the successful expansion of ivacaftor, the idea is to keep testing cells to find out whether other mutations respond to existing modulators, with plans to study new modulators as they are approved. In a video played at the plenary, we heard how critical this type of work is from Callie Dolan, an adult with CF who benefitted from the FDA's recent decision.
Currently, there are approximately 650 rare mutations that result in a defective CFTR protein that may be corrected by a modulator and are therefore very good prospects for theratyping. Testing is already underway at three Foundation-funded labs to see if these mutations respond to current modulators. If any of the tests show positive results, the Foundation plans to work with drug companies to see if those mutations can be approved for those drugs. We are hoping that Hailey Huyser, who was also in the video, will be one of the people who will benefit from this type of testing.
We're supporting a number of academic investigators who are working on different parts of the theratyping initiative. As of June 2017, we had $1.5 million in grants for theratyping, with more expected to be funded in the coming year.
We also continue to make progress toward developing potential therapies for the 5 percent of people with CF who have mutations that cannot be treated solely with a modulator. At labs across the country, we are aggressively pursuing cutting-edge approaches -- like RNA therapy and readthrough compounds -- to develop treatments for people with CF with these rare and nonsense mutations. In addition, half of the research being performed at the Cystic Fibrosis Foundation Therapeutics (CFFT) Lab is focused on these types of therapies.
The lab's scientists are also spearheading a new initiative to collect and grow cells from people with rare mutations. These cells will be critical for screening studies to identify new potential drugs and other treatment options for rare mutations. CFFT will be collecting these cells at sites around the U.S. as part of an observational study called RARE.
Until we can find a cure, we will continue to do whatever we can to get life-changing medications safely and quickly to as many people with CF as we can. I'm looking forward to an exciting 2018.
Watch the recording of plenary 1, Matching Medicines With Mutations, in its entirety on YouTube.